
Project Management Software
Oct 28th, 2025
In the pharmaceutical business, every business decision costs billions. Here delays mean lost months, or even years, of patient access to life-saving drugs. The pressure on pharma business owners, project managers, and executive decision-makers is huge. This stress comes from a development process that is complex, expensive, and high-risk.
The old ways of managing projects no longer work in the ever-evolving pharmaceutical industry. Using spreadsheets, email chains, and siloed departmental databases is not enough. Regulators, investors, and the global public demand better.
To succeed, companies must stop just “doing projects.” They must manage their entire drug pipeline as structured, connected projects. This shift needs a dedicated Project Management System (PMS). A PMS is more than a digital planner. It is a validated framework. It enforces necessary structure and transparency. This spans the whole organization, from the lab bench through to the commercial launch.
The real benefit of a Project Management System in pharma isn’t saving money on software. It’s about accelerating drug delivery to the market. It ensures unshakeable compliance. Most importantly, it protects multi-billion dollar investments.
Before discussing the benefits, we must acknowledge the huge industry challenges. Insufficient project governance makes these problems much worse.
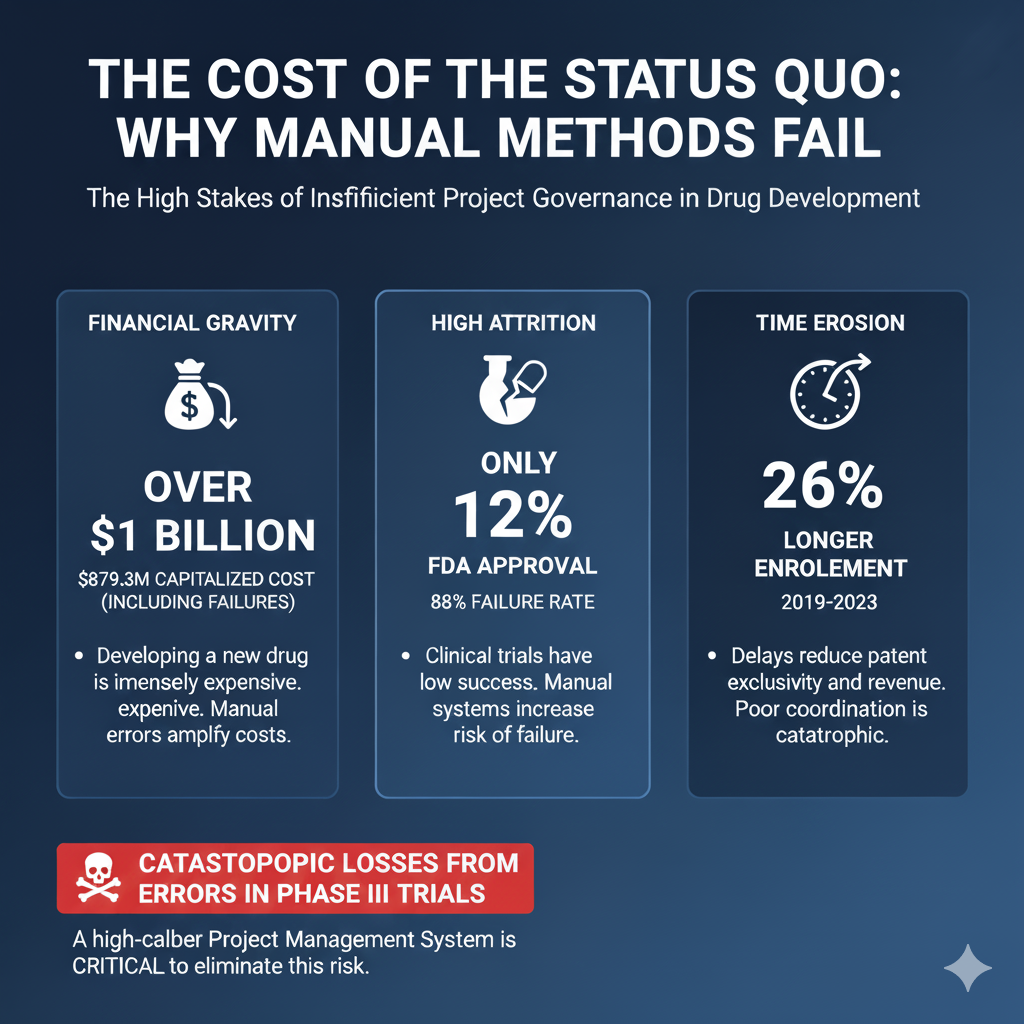
A Phase III clinical trial costs hundreds of millions of dollars. If it fails due to documentation errors or a missed regulatory date, the losses are enormous. A high-caliber Project Management System is designed precisely to eliminate this critical risk.
Compliance is the most critical project goal in pharma. Speed comes second. When you fail to meet Good Manufacturing Practice (GMP), Good Clinical Practice (GCP), and Good Laboratory Practice (GLP), it will affect the product launch negatively. A Project Management System (PMS) helps you change compliance. It moves it from a reactive burden to a proactive, built-in workflow.
Regulators like the FDA and EMA follow a strict rule: “If it isn’t documented, it didn’t happen.” This makes a PMS vital for project management in pharmaceutical industry.
The PMS gives Quality and Regulatory leaders instant readiness. The organization can move from development to a successful inspection. This avoids the frantic, last-minute search for data.
The market race requires exact schedules. It also needs optimal use of costly, specialized resources. A good Project Management System (PMS) is key here. It powerfully optimizes both resources and timelines.
Drug development involves complex hand-offs. Teams include research (preclinical), clinical (trial phases), manufacturing (CMC), and regulatory affairs. A delay in one phase can cascade. For instance, slow Phase II enrollment causes multi-month project overruns.
Operationally, a PMS works like advanced Task Management Software. This applies in the lab or the clinical research office.
Picture a large, global Phase III trial. It involves many sites. The system breaks the trial into thousands of trackable tasks:
This granular control is vital. Platforms like TaskOPad offer intuitive dashboards and automated reminders. This ensures every team member knows their exact duties and deadlines. It increases accountability. It keeps local work aligned with global strategy.
Owners and CFOs view R&D spending as an investment. Complex projects often face cost overruns. Strong financial control is vital. A Project Management System (PMS) offers the visibility needed to protect the R&D budget.
Scientific staff, key equipment (like bioreactors), and clinical sites are top company assets. They are also highly constrained. Overloading a key scientist wastes efficiency. Underutilizing expensive equipment hits the budget directly.
Every pharma project faces potential risks including unexpected side effects, vendor issues, supply chain breaks, or new regulations.
The pharmaceutical pipeline is the business engine. A Project Management System (PMS) makes project management a strategic asset. It moves beyond mere operations.

The hand-off is difficult. The clinical team focuses on scientific rigor. The commercial team focuses on market access and sales. Miscommunication is common here. Project Management Software offers one central source of truth. It unites these different groups.
A powerful PMS formalizes processes. It provides real-time visibility. This empowers your leadership team to:
For an organization dedicated to innovation and patient health, the shift to a structured Project Management System is inevitable. It is the necessary evolution for an industry defined by complexity.
The choice is whether to continue managing multi-billion dollar projects with legacy tools that hide risks and encourage manual error, or to adopt a validated, scalable solution that instills structure across the entire value chain.
Look for a Project Management Software solution like TaskOPad that is designed with user clarity and regulatory robustness in mind. The ideal system should offer flexible deployment, intuitive Task Management Software features, and the scalability to manage not just one clinical trial, but an entire portfolio of R&D, manufacturing, and commercial projects.
Embracing this transformation is not merely about achieving operational efficiency; it is about building a future-proof, compliant, and market-leading pharmaceutical enterprise. Book your free demo and see how TaskOPad can completely transform your workflow with a feature-rich project management system.
Search by posts
Search by posts
Recent posts
12-31-2025
Cloud Based Project Management Software
12-30-2025
Task Management Software
12-29-2025
Project Management For Managers
12-29-2025
Project Management
12-27-2025
Office Management Software
© Copyright 2026 Taskopad Solutions Private Limited. All rights reserved.